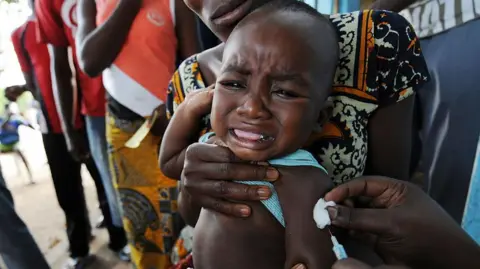
WHO Condemns Controversial US-Funded Vaccine Trial in Guinea-Bissau
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization as 'unethical'.
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age.
The WHO said it had 'significant concerns' about the plan, calling the birth-dose vaccine an 'effective and essential public health intervention, with a proven record'.
The trial, primarily led by Danish researchers and sponsored by the US Centers for Disease Control and Prevention (CDC), was intended to explore the extended health effects of the vaccine, despite being in a country where significant numbers are affected by hepatitis B.
The US health department, led by Robert F Kennedy Jr., who has been known to question vaccine effects, had used the trial to address broader health concerns linked to the hepatitis B jab.
The WHO emphasized the need for ethical safeguards and scientific justification in conducting this research. It pointed out that giving a proven life-saving intervention to some newborns but not others could expose them to 'potentially irreversible harm'.
WHO officials noted that hepatitis B vaccination administered at birth prevents the virus's transmission from mother to baby in 70-95% of cases. With a considerable portion of Guinea-Bissau's population estimated to be infected, the organization underscored the necessity of immediate vaccination at birth.
Despite criticism leading to the trial's suspension, the Guinea-Bissau government is moving to introduce the birth dose nationwide by 2028, aligning with global vaccination standards.
Public opinion has turned against the trial, with prominent figures in Guinea-Bissau, including former health minister Magda Robalo, expressing that locals should not serve as test subjects. She remarked that 'Guinea-Bissauans are not guinea pigs'.
The WHO stresses that ethical trials must have proven treatments available, which is not the case here considering the widely accepted efficacy of the hepatitis B vaccine, used for over 30 years in more than 115 countries.