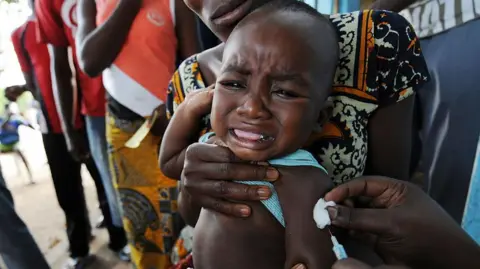
Controversy Erupts Over US-Funded Vaccine Trial in Guinea-Bissau
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization as unethical. The US-funded study sought to administer the vaccine at birth to one group of infants, while another group would receive it six weeks later.
The WHO expressed significant concerns regarding the scientific justification for the trial, ethical safeguards, and adherence to established standards for human research. They highlighted the importance of the birth-dose vaccine as a vital public health intervention.
Led by the US health department under Robert F. Kennedy Jr., who has been openly critical of vaccines, the trial aimed to answer questions about the broader health impacts of the hepatitis B vaccine.
Following public outcry, the Guinea-Bissau government suspended the trial last month, reflecting widespread skepticism about the ethics of using local newborns for such research.
Critics argue that giving a life-saving vaccine to some while withholding it from others poses a risk of potentially irreversible harm. The WHO reiterates that vaccination at birth reduces the risk of mother-to-child transmission by 70-95%. In Guinea-Bissau, where a significant portion of the population is affected by hepatitis B, the WHO advocates for administering the hepatitis B vaccine to all newborns within 24 hours of birth.
Currently, the vaccine is given at six weeks, but there are plans to transition to the birth dose by 2028. With around 14,000 newborns expected to participate in the halted study, many remain skeptical about the ethical implications of such trials in regions where effective vaccines already exist. As public discourse continues, the voices of local health advocates emphasize the need for ethical practices in vaccine distribution and research.