Limited recall due to mix-up offers precautionary advice for users and handback instructions
Recall of Yaz Plus Contraceptive Pill in South Africa Due to Packaging Error
Recall of Yaz Plus Contraceptive Pill in South Africa Due to Packaging Error
Bayer Ltd warns women about potentially ineffective birth control pills from affected batch
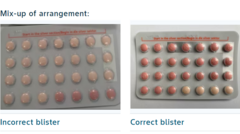
The South African health authorities have announced a recall of a specific batch of the widely-used Yaz Plus contraceptive pill after it was discovered that some packages contained the wrong number of active pills. The mix-up, noted by Bayer Ltd, is particularly concerning as it could render the contraception ineffective for women who believe they are taking the right dosage.
Women in possession of blister packs labeled WEW96J, with an expiration date of March 2026, are urged to stop using them immediately and consult with healthcare providers. Many of these affected packages mistakenly contain 24 inactive pills instead of the necessary active hormone pills. This inconsistency could pose a risk of unintended pregnancy among users who assume they are taking effective contraceptives.
In collaboration with the South Africa Health Products Regulatory Agency, Bayer Ltd has issued a recall, emphasizing the importance of not using any tablets from this batch until professional medical advice is sought. The company has clarified that the issue pertains only to this limited batch, with corrective measures already implemented to prevent future occurrences.
Consumers who have obtained a packet from this specific batch should return the pills to their pharmacies for a replacement or refund. Furthermore, healthcare professionals, including hospitals and pharmacies, are also instructed to return any remaining stock of the affected pills. Bayer has established a helpline for individuals seeking additional information regarding the recall, underscoring their commitment to women's health safety in light of this packaging error.
Women in possession of blister packs labeled WEW96J, with an expiration date of March 2026, are urged to stop using them immediately and consult with healthcare providers. Many of these affected packages mistakenly contain 24 inactive pills instead of the necessary active hormone pills. This inconsistency could pose a risk of unintended pregnancy among users who assume they are taking effective contraceptives.
In collaboration with the South Africa Health Products Regulatory Agency, Bayer Ltd has issued a recall, emphasizing the importance of not using any tablets from this batch until professional medical advice is sought. The company has clarified that the issue pertains only to this limited batch, with corrective measures already implemented to prevent future occurrences.
Consumers who have obtained a packet from this specific batch should return the pills to their pharmacies for a replacement or refund. Furthermore, healthcare professionals, including hospitals and pharmacies, are also instructed to return any remaining stock of the affected pills. Bayer has established a helpline for individuals seeking additional information regarding the recall, underscoring their commitment to women's health safety in light of this packaging error.


















