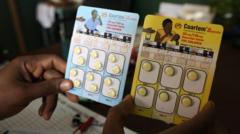
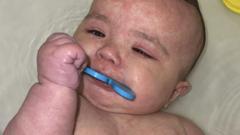
The newly approved solution, developed by Novartis in collaboration with global health organizations, aims to bridge this critical treatment gap. Known as Coartem Baby or Riamet Baby, the drug has been optimized for the youngest patients, offering a lifeline for millions of vulnerable children.
Novartis announced its commitment to distribute the drug on a not-for-profit basis as experts emphasize its crucial role in public health. The launch will begin in African countries hardest hit by malaria, marking a significant advancement in the global fight against this deadly disease.
In a statement, Novartis CEO Vas Narasimhan expressed pride in this achievement. The drug was developed alongside the Medicines for Malaria Venture (MMV), supported by various international bodies. MMV CEO Martin Fitchet highlighted the importance of this treatment in combating malaria’s impact on children. Meanwhile, health experts are optimistic that the introduction of Coartem Baby will drastically reduce mortality rates associated with the disease in infants, particularly in sub-Saharan Africa, where the crisis is most severe.
This promising treatment demonstrates the potential to address health inequalities and improve access to essential care for the world's most vulnerable populations.
Novartis announced its commitment to distribute the drug on a not-for-profit basis as experts emphasize its crucial role in public health. The launch will begin in African countries hardest hit by malaria, marking a significant advancement in the global fight against this deadly disease.
In a statement, Novartis CEO Vas Narasimhan expressed pride in this achievement. The drug was developed alongside the Medicines for Malaria Venture (MMV), supported by various international bodies. MMV CEO Martin Fitchet highlighted the importance of this treatment in combating malaria’s impact on children. Meanwhile, health experts are optimistic that the introduction of Coartem Baby will drastically reduce mortality rates associated with the disease in infants, particularly in sub-Saharan Africa, where the crisis is most severe.
This promising treatment demonstrates the potential to address health inequalities and improve access to essential care for the world's most vulnerable populations.