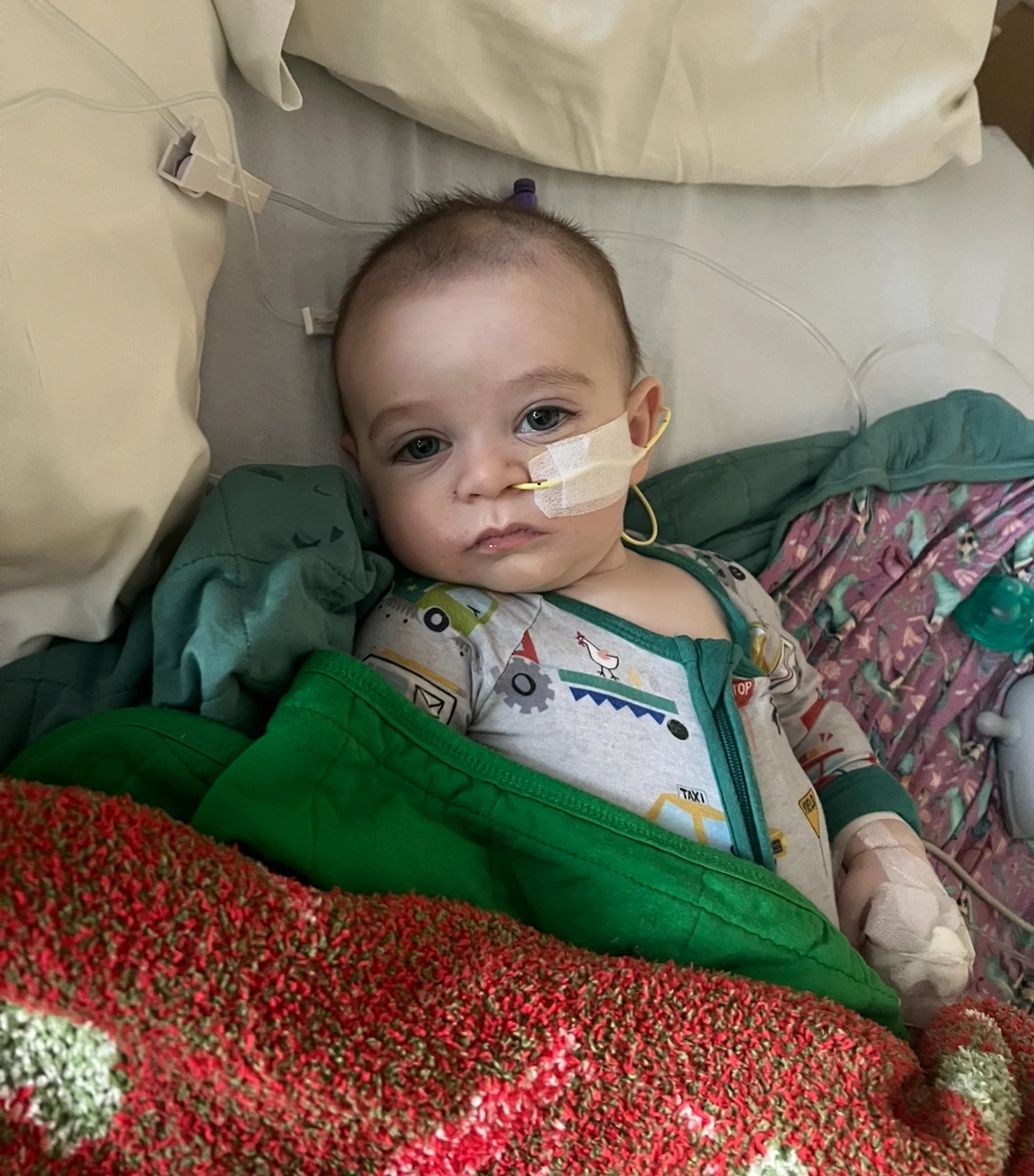
Tests of ByHeart infant formula tied to a botulism outbreak that has sickened dozens of infants showed that all of the company’s products may have been contaminated.
Laboratory tests on 36 samples from three production lots determined that five samples contained bacteria capable of inducing the rare and potentially deadly illness. In a statement released Monday, the company acknowledged the potential risk and advised caution.
As of now, 31 babies across 15 states have been reported sick after consuming ByHeart formula since the outbreak began in August. Notably, some infants were treated for botulism as far back as November 2024, though those cases are not counted in the current outbreak statistics.
Clostridium botulinum type A, the bacteria found, can be unevenly distributed in powdered formulas. Medical professionals warn that not all infants consuming the contaminated formula will fall ill; however, all infants under the age of one remain at risk.
ByHeart initiated a nationwide recall of its formula on November 11. Unfortunately, some products remain on store shelves despite this recall, as noted by state officials and the FDA.
Parents are strongly advised to stop using ByHeart formula and monitor for symptoms, which may take up to 30 days to manifest. Symptoms of botulism include constipation, difficulties with sucking or feeding, drooping eyelids, a flat facial expression, and weakness.
As the situation evolves, it is crucial for parents to report any illnesses linked to the outbreak to an FDA consumer complaint coordinator or through an online MedWatch form.
If you purchased ByHeart formula on or after August 1, you can obtain a full refund by contacting the company directly.