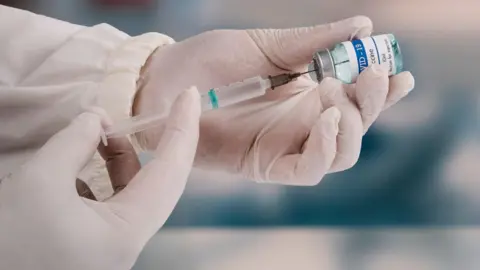
In a significant policy shift, the US Department of Health and Human Services (HHS), under Health Secretary Robert F. Kennedy Jr., will discontinue $500 million (£376 million) in funding aimed at mRNA vaccine development targeting respiratory viruses such as flu and Covid-19. This decision affects 22 initiatives led by prominent pharmaceutical firms including Pfizer and Moderna, as confirmed by HHS.
Kennedy, known for his vaccine skepticism, justified the funding cancellation by asserting that "mRNA technology poses more risks than benefits for these respiratory viruses." His stance has been met with substantial backlash from medical professionals who argue that questioning vaccine safety undermines public health.
Peter Lurie, a former official with the US Food and Drug Administration, emphasized the importance of mRNA vaccines in mitigating the pandemic, stating that the country's decision represents a rejection of a vital tool in combatting future health crises. Kennedy responded by asserting that his administration's review concluded these vaccines do not offer adequate protection against respiratory diseases.
The Health Secretary also claimed mRNA vaccines could foster new viral mutations, extending pandemic conditions due to the virus adapting to evade vaccine-induced immunity. However, experts have rebutted this claim, explaining that viruses inherently mutate, making vaccination a necessary preventative measure. Dr. Paul Offit, from the Vaccine Education Center, described mRNA vaccines as safe and a critical line of defense against severe infections, intensifying concerns that this funding cut could hinder future pandemic responses.
The HHS plans to allocate its resources to "safer, broader vaccine platforms" with proven safety records instead. Notably, while mRNA vaccines operate by instructing cells to produce immune response-triggering proteins, traditional vaccines often rely on inactivated viruses, which may not offer the same rapid development advantages.
Kennedy's recent policy changes also include the dismissal of the entire vaccine advisory committee and alterations to immunization recommendations, raising apprehensions over the future of vaccine regulation in the US. The latest developments suggest a pivot away from innovative vaccine technology at a time when global health scientists stress the importance of preparedness against potential future pandemics.
Kennedy, known for his vaccine skepticism, justified the funding cancellation by asserting that "mRNA technology poses more risks than benefits for these respiratory viruses." His stance has been met with substantial backlash from medical professionals who argue that questioning vaccine safety undermines public health.
Peter Lurie, a former official with the US Food and Drug Administration, emphasized the importance of mRNA vaccines in mitigating the pandemic, stating that the country's decision represents a rejection of a vital tool in combatting future health crises. Kennedy responded by asserting that his administration's review concluded these vaccines do not offer adequate protection against respiratory diseases.
The Health Secretary also claimed mRNA vaccines could foster new viral mutations, extending pandemic conditions due to the virus adapting to evade vaccine-induced immunity. However, experts have rebutted this claim, explaining that viruses inherently mutate, making vaccination a necessary preventative measure. Dr. Paul Offit, from the Vaccine Education Center, described mRNA vaccines as safe and a critical line of defense against severe infections, intensifying concerns that this funding cut could hinder future pandemic responses.
The HHS plans to allocate its resources to "safer, broader vaccine platforms" with proven safety records instead. Notably, while mRNA vaccines operate by instructing cells to produce immune response-triggering proteins, traditional vaccines often rely on inactivated viruses, which may not offer the same rapid development advantages.
Kennedy's recent policy changes also include the dismissal of the entire vaccine advisory committee and alterations to immunization recommendations, raising apprehensions over the future of vaccine regulation in the US. The latest developments suggest a pivot away from innovative vaccine technology at a time when global health scientists stress the importance of preparedness against potential future pandemics.