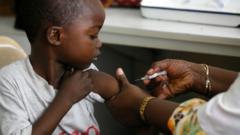
In a groundbreaking development, a new malaria treatment designed explicitly for infants and very young children has received approval for use. This new medication is expected to launch in African countries in the coming weeks, filling a crucial gap in malaria treatment for the most vulnerable demographic—infants who weigh less than 4.5 kilograms (around 10 pounds).
Until now, babies have been subjected to medications formulated for older children, which can be dangerously dosed and potentially lead to harmful overdose situations. In 2023, the malaria epidemic contributed to approximately 597,000 deaths, with a significant number of these fatalities occurring in Africa, predominantly among children under five years old.
The newly approved drug, produced by Novartis, aims to address the “treatment gap” previously experienced in this age group. The chief executive of Novartis, Vas Narasimhan, emphasized the importance of this milestone, stating, "For more than three decades, we have remained committed to the fight against malaria and are proud to now deliver the first clinically proven treatment for newborns and young babies."
Known as Coartem Baby or Riamet Baby in various regions, this treatment was developed in collaboration with the Medicines for Malaria Venture (MMV), a global non-profit organization supported by several governments and major foundations. The approval process included involvement from eight African nations, which are set to be among the first to utilize this life-saving drug.
Experts are optimistic that the introduction of Coartem Baby will significantly impact malaria-related mortality among infants. Martin Fitchet, CEO of MMV, noted the potential of this drug to change the trajectory of malaria treatment for neglected patients. "Malaria is one of the world's deadliest diseases, particularly among children," he said. "With the right resources and focus, it can be eliminated."
Dr. Marvelle Brown from the University of Hertfordshire characterized the approval as a monumental step toward saving young lives, especially given that over 76% of malaria-related deaths occur in children under five years old. He also highlighted the added risks posed to infants with underlying health conditions such as sickle cell disease.
The decision by Novartis to offer this medication on a not-for-profit basis is expected to enhance access to the treatment and reduce healthcare inequalities throughout affected regions.